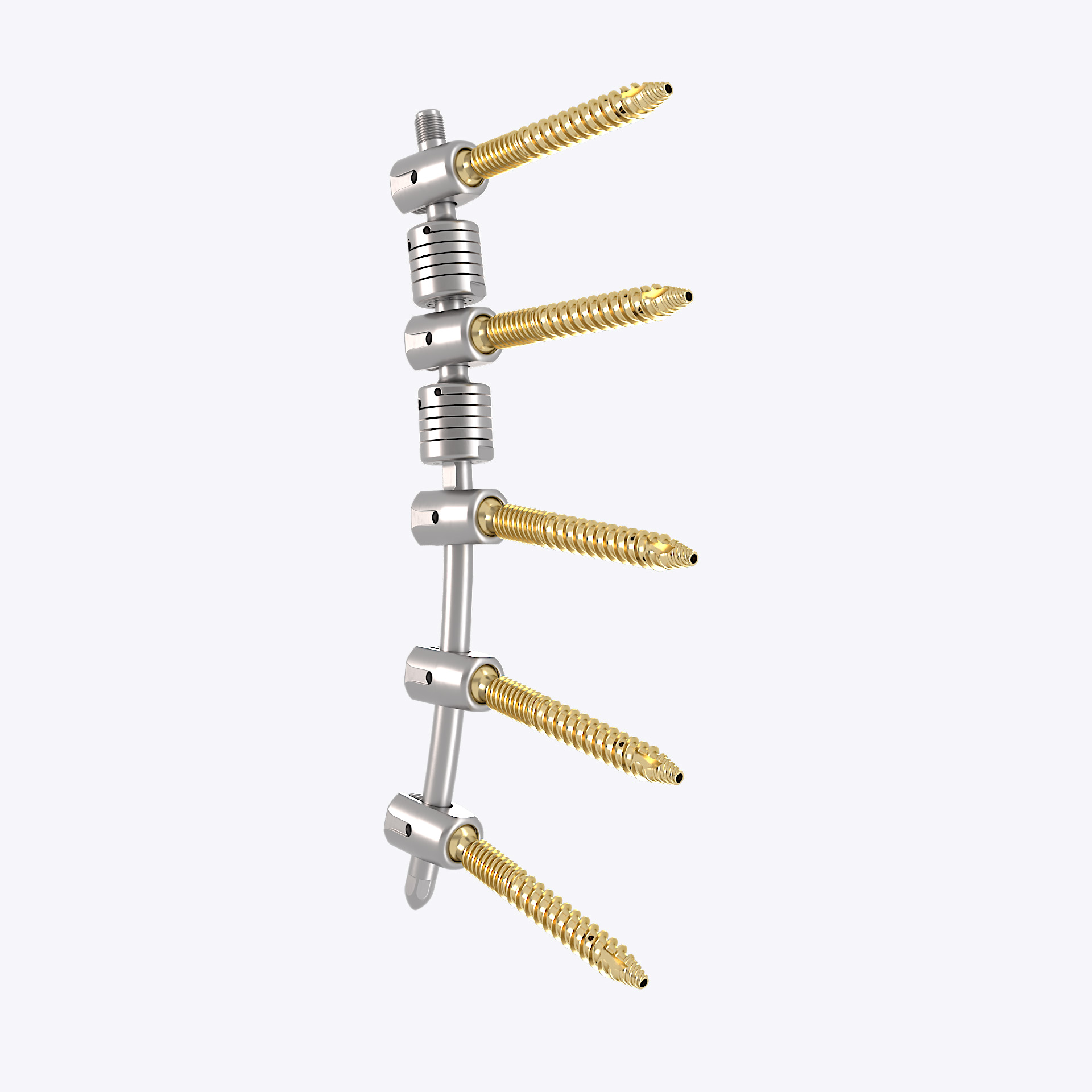
The modular HPS™ Hybrid Performance System is used for single or multi-segmental posterior stabilization of the thoracolumbar spine. The system is based on fixation with polyaxial pedicle screws. The system allows both dynamic and rigid stabilization. The HPS™ Pedicle screw, fenestrated is also used for single or multi segmental posterior rigid stabilization of the thoracolumbar spine in combination with bone cement.
Intended Purpose
The HPS™ Hybrid Performance System is a pedicle screw-based implant system for posterior dynamic stabilization and/or for posterior rigid stabilization of the thoracolumbar spine in skeletally mature patients. The HPS is intended for long-term implantation and for single-use only. It is delivered sterile and is not intended for re-processing by the user or third parties. The HPS™ Hybrid Performance System shall only be used by surgeons who are trained and familiar with the implant components, instruments, and surgical technique.
Implantation Parameters
The HPS™ 2.0 Hybrid Performance System as a system for posterior dynamic stabilization is intended for posterior dynamic stabilization at one to three contiguous spinal segments from L1 to S1 after (micro-) surgical decompression in the treatment of degenerative spinal stenosis including degenerative spondylolisthesis (pseudospondylisthesis) up to Meyerding grade 1, which were previously unsuccessfully conservatively treated.
Posterior Rigid Stabilization
The HPS™ 2.0 Hybrid Performance System as a posterior rigid stabilization system is intended for posterior rigid stabilization of the thoracolumbar spine as adjunct to fusion for the following indications:
- degenerative disc disease (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies)
- spondylolisthesis
- trauma (i.e., fracture or dislocation)
- spinal stenosis
- curvatures (i.e., kyphosis, and/or lordosis)
- tumor
- pseudoarthrosis
- and failed previous fusion
HPS™ Pedicle screw, fenestrated
The HPS™ Pedicle screw, fenestrated, is indicated for the use with bone cement in patients with diminished bone quality (e.g. osteoporosis, osteopenia, metastatic disease) and shall only be used with components for posterior rigid stabilization of the HPS™ Hybrid Performance System for mono- or multisegmental stabilization of the thoracic and lumbar spine for supporting fusion.
Material
The Hybrid Performance System is made from Titanium 6 aluminum 4-vanadium alloy (ISO 5832-3).
Surgical Technique Guide
Please access the current Surgical Technique Manuals for detailed step-by-step guidance on the proper handling and implantation of our devices. These manuals are intended for trained medical professionals only. The HPS™ 2.0 implant is intended to be used exclusively in combination with the designated HPS™ 2.0 instruments. The correct and safe use of the implant is ensured only when the associated surgical instruments specified for the HPS™ 2.0 system are utilized. The complete list and description of these designated instruments are provided in the HPS™ 2.0 Surgical Technique Guide (STG-00069 Rev A / STG-00070 Rev A ). This guide outlines the appropriate surgical steps, instrumentation, and procedures necessary for safe and effective implantation and removal.
The Surgical Technique Guides (STG-00069 Rev A / STG-00070 Rev A) is available below :
Summary of Safety and Performance (SSCP)
In accordance with the EU Medical Device Regulation (MDR, Regulation (EU) 2017/745), access to the Summary of Safety and Clinical Performance (SSCP) is provided for applicable devices. The SSCP is intended to give healthcare professionals and, where relevant, patients clear, updated information on the safety, clinical benefits, and performance of the device. The document can be requested by contacting our Customer Service department. Please contact us at psg-service@paradigmspine.com for additional details or support.
Implant Cards
In line with EU MDR (Regulation (EU) 2017/745) requirements, all Paradigm Spine implants are supplied with an Implant Card for patients. Please ensure that each patient receives their Implant Card after implantation and understands its purpose. The Implant Card helps patients identify the implanted device and provides key information for future reference and medical care. The Implant Card must be completed with the relevant patient and implant information and handed over to the patient. The implant card is being provided with the implant but can additionally be requested via email at psg- service@paradigmspine.com. For more detailed information please refer to our Implant Card Instructions:
Patient Information
At Paradigm Spine, we believe that clear information helps you make the best decisions about your health. Here you can find our Patient Information Leaflets, which explain our products, how they work, and what you can expect before and after treatment. Please download the leaflet for detailed information about your device, how it is used, and answers to common questions. If you have any further questions, please contact your healthcare professional or reach out to us directly — we are here to help.
The HPS™ 2.0 Patient Information Leaflet offers essential information about the implanted device, its purpose, and guidance for post-operative behavior. This information can be obtained from our Customer Service team, who will be happy to assist you. Please contact us at psg-service@paradigmspine.com for additional details or support.
Implant Cards
The Implant Card must be completed with the relevant patient and implant information and handed over to the patient. For more detailed information please refer to our Implant Card Instructions:
Summary of Safety and Performance (SSCP)
The SSCP is intended to provide patients clear, updated information on the safety, clinical benefits, and performance of the device. This information can be obtained from our Customer Service team, who will be happy to assist you. Please contact us at psg-service@paradigmspine.com for additional details or support.