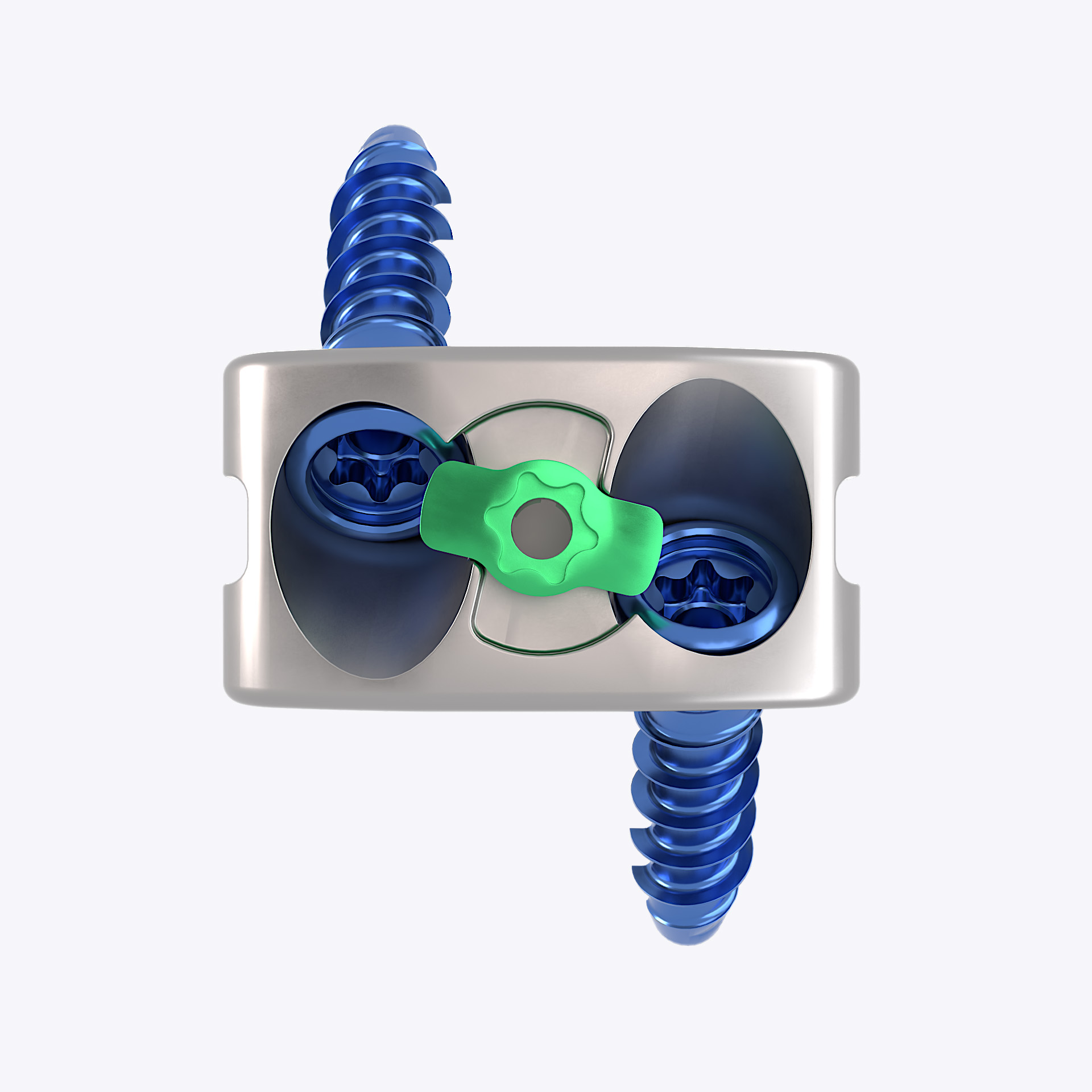
The Fortilink®-SC Ti is a stand-alone interbody fusion device intended for the cervical spine (C2-T1) in patients with degenerative disc diseases. The Fortilink®-SC Ti is composed out of a cage/wedge, two screws and a retainer. The Fortilink®-SC Ti interbody fusion devices are manufactured with SLM (selective laser melting) and are built up from implant grade titanium alloy (Ti6Al4V).
The Fortilink®-SC Ti has an open mesh structure and a bone window both designed to allow bone ingrowth and facilitate fusion. The box-shaped design is intended to provide primary stability and increase the intervertebral height and lordosis. The screws provide primary stability to facilitate fusion and supplemental fixation is therefore not required. The retainer locks the screws. Both the screws and the retainer are made from titanium alloy.
The Fortilink®-SC Ti will be used in combination with:
• Dedicated instrument set (see surgical technique for Catalog Instruments)
• General instruments typically used in spinal surgery (including rongeurs, forceps).
Intended Purpose
The Fortilink®-SC Ti is indicated for stand-alone anterior cervical interbody fusion procedures in skeletally mature patients with degenerative disc disease (DDD) at one or two levels from C2 to T1. Degenerative disc disease is defined as neck pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies.
The system is intended to be used with autograft and/or allogenic bone graft comprised of cancellous and/or corticocancellous bone graft to facilitate fusion and is implanted via an anterior approach. Implants must be used with two of the provided bone screws. This system is to be used in patients who have had six weeks of non-operative treatment.
Implantation Parameters
Intended Body Region: Cervical spine (C2 to T1)
Patient Population: Skeletally mature patients with degenerative disc disease (DDD) and spinal instabilities, including those with degenerative disc disease defined as neck pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies.
Intended Benefits:
- Decrease neck pain, as assessed using the Visual Analogue Scale (VAS)
- Clinical improvement, as assessed using the Neck Disability Index (NDI) and the Japanese Orthopaedic Association (JOA).
- Facilitate fusion, assessed by fusion rates in radiological follow-up images.
- Increase in intervertebral height, as measured by increase in post-operative disc height on radiological imaging.
- Increase in lordosis, as measured by increase in post-operative lordosis on radiological imaging
Material
The implants are manufactured from implant grade titanium alloy Ti6Al4V ELI (ASTM F3001).
The screws and retainers are made of implant grade titanium alloy Ti6Al4V ELI (ASTM F136).
Surgical Technique Guide
The Surgical Technique Guide is available here:
End User Information - BAAT Medical | Full Service Device Development
Summary of Safety and Performance (SSCP)
In accordance with the EU Medical Device Regulation (MDR, Regulation (EU) 2017/745), access to the Summary of Safety and Clinical Performance (SSCP) is provided for applicable devices. The SSCP is intended to give healthcare professionals and, where relevant, patients clear, updated information on the safety, clinical benefits, and performance of the device. The document can be requested by contacting our Customer Service department. Please contact us at psg-service@paradigmspine.com for additional details or support
Patient Information
At Paradigm Spine, we believe that clear information helps you make the best decisions about your health. Here you can find our Patient Information resources, which explain our products, how they work, and what you can expect before and after treatment.
If you have any further questions, please contact your healthcare professional or reach out to us directly — we are here to help. Please contact us at psg-service@paradigmspine.com for additional details or support.
Summary of Safety and Performance (SSCP)
In accordance with the EU Medical Device Regulation (MDR, Regulation (EU) 2017/745), access to the Summary of Safety and Clinical Performance (SSCP) is provided for applicable devices.
The SSCP is intended to give healthcare professionals and, where relevant, patients clear, updated information on the safety, clinical benefits, and performance of the device. The document can be requested by contacting our Customer Service department. Please contact us at psg-service@paradigmspine.com for additional details or support