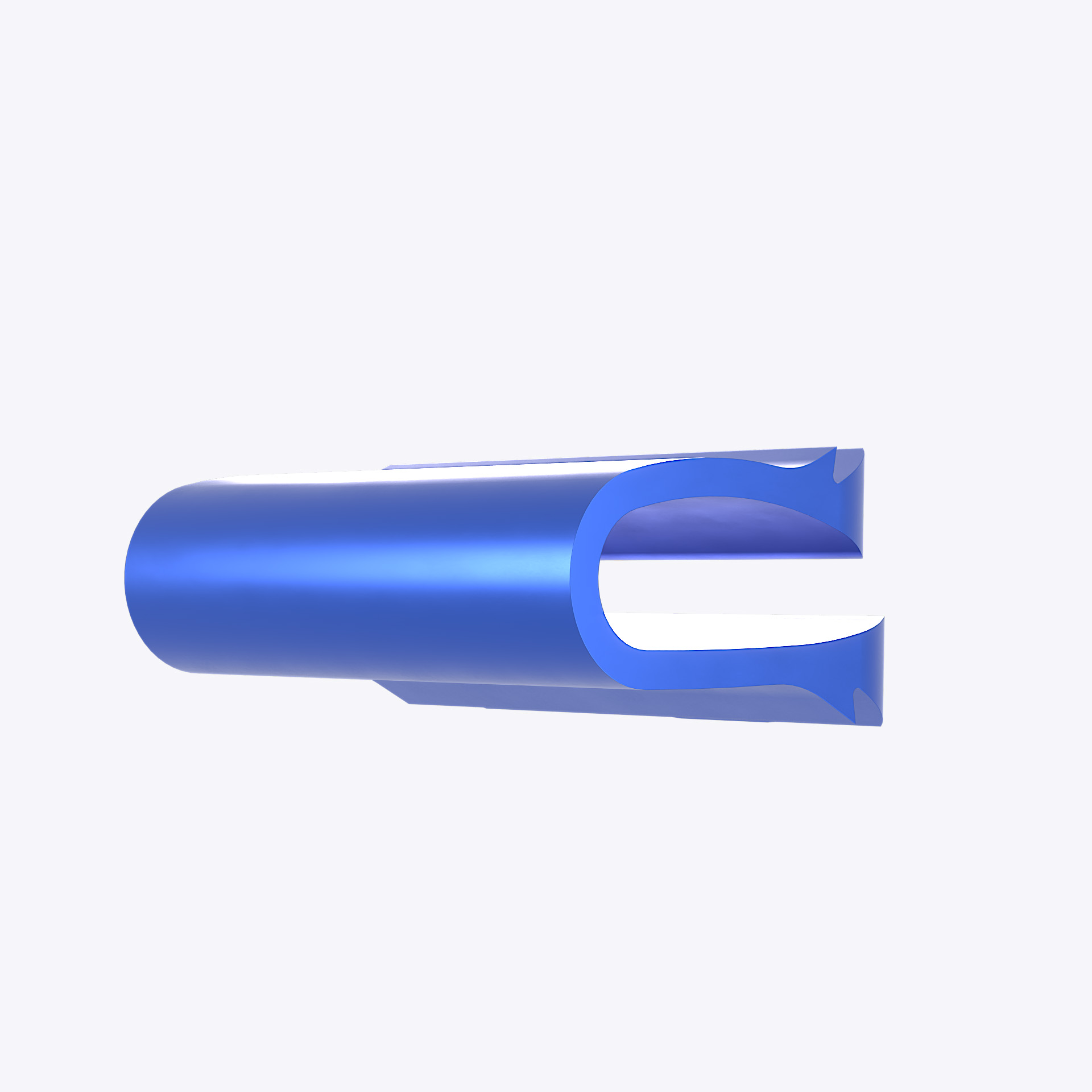
DCI™ implant
The DCI™ is a dynamic implant intended for implantation in the anterior cervical disc space (C3-C7) of patients with cervical disc herniation, cervical DDD, cervical Stenosis, with or without myeloradiculopathy in patients with or without neck pain. The DCI™ provides stable and controlled motion, by allowing flexion and extension relative to the neutral position. The implants are long-term implantable devices designed in a U-shape to fit into the anatomical geometry of the disc space in the cervical spine.
Intended Purpose
The DCI™ implant is intended for implantation in the anterior cervical disc space at 1 to 3 levels from C3 to C7 in skeletally mature patients. It controls segmental motion in cases of cervical disc herniations, cervical degenerative disc dis-ease (DDD) and cervical canal stenosis, with or without myeloradiculopathy.
Implantation Parameters
The DCI™ device is intended to be implanted in the intervertebral disc space of one to three cervical spinal segments from C3 to C7. A maximum of three (3) DCI™ implants may be implanted per patient. Each implant is intended for use at a single cervical motion segment, and the DCI™ system is approved for treatment of up to three (3) cervical motion segments (C3–C7).
Material
The DCI™ implant is made from wrought titanium 6-aluminum 4 vanadium alloy (ISO 5832-3). The used material contains no nickel and is not made with natural rubber latex.
Surgical Technique Guide
The Surgical Technique Guide (STG-00019) is available below :
Summary of Safety and Performance (SSCP)
In accordance with the EU Medical Device Regulation (MDR, Regulation (EU) 2017/745), Paradigm Spine provides access to the Summary of Safety and Clinical Performance (SSCP) for applicable devices. The SSCP is intended to give healthcare professionals and, where relevant, patients clear, updated information on the safety, clinical benefits, and performance of the device.
Please refer to the link below to view or download the current SSCPs:
Implant Cards
In line with EU MDR (Regulation (EU) 2017/745) requirements, all Paradigm Spine implants are supplied with an Implant Card for patients. Please ensure that each patient receives their Implant Card after implantation and understands its purpose. The Implant Card helps patients identify the implanted device and provides key information for future reference and medical care. The Implant Card must be completed with the relevant patient and implant information and handed over to the patient. The implant card is being provided with the implant but can additionally be requested via email at psg- service@paradigmspine.com. For more detailed information please refer to our Implant Card Instructions:
Patient Information
At Paradigm Spine, we believe that clear information helps you make the best decisions about your health. Here you can find our Patient Information Leaflets, which explain our products, how they work, and what you can expect before and after treatment. Please download the leaflet for detailed information about your device, how it is used, and answers to common questions. If you have any further questions, please contact your healthcare professional or reach out to us directly — we are here to help.
The DCI™ Patient Information Leaflet offers essential information about the implanted device, its purpose, and guidance for post-operative behavior. This document is available below or can be requested by email at psg-service@paradigmspine.com.
Implant Cards
The Implant Card must be completed with the relevant patient and implant information and handed over to the patient. For more detailed information please refer to our Implant Card Instructions:
Summary of Safety and Performance (SSCP)
The SSCP is intended to patients clear, updated information on the safety, clinical benefits, and performance of the device. Please refer to the link below to view or download the current SSCPs: